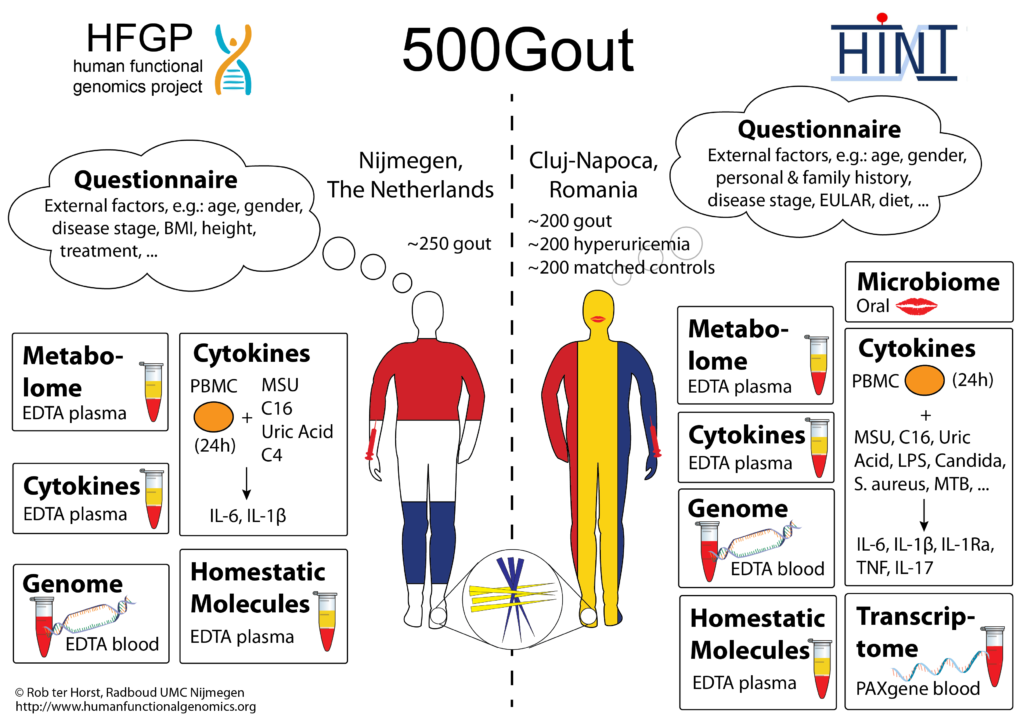
Uric acid is a metabolite formed as a breakdown product of purines and is regarded as an endogenous danger signal released during cellular stress. Hyperuricaemia is defined as the elevation of serum uric acid levels above the threshold of 0.36 mM when uric acid crystallization ensues. Up to now, major lines of research investigating the pro-inflammatory effects of uric acid have focused on monosodium urate crystal-induced processes. However, emerging data suggest that uric acid in soluble form might also have pro-inflammatory effects. Recently, our group has discovered that uric acid also exerts pro-inflammatory properties through a direct effect on human primary peripheral blood mononuclear cells (PBMC)s. We have found that PBMCs of patients with hyperuricemia produce higher amounts of pro-inflammatory cytokines than healthy controls after ex-vivo stimulation, but the mechanisms responsible for this inflammatory dysbalance are not known. Of high interest, gout patients that were treated with uric acid lowering compounds reduced their capacity to produced pro-inflammatory cytokines ex-vivo. In line with this, when human primary monocytes were primed in-vitro with high uric acid concentrations for 24h, followed by restimulation for 24h with TLR ligands, we noted strongly elevated cytokine responses. These effects were long lived, and this metabolic imprinting seems to be mediated by epigenetic reprogramming, because inhibitors of histone methyl transferases abolished the “reprogramming” effect of uric acid. These data suggest that uric acid is a danger signal capable to modify inflammatory cells through epigenetic modification, a process reminiscent of the ‘trained immunity’ process recently described in infections and vaccinations.